North America and Europe PEGylated Drugs Market to witness massive growth from cancer treatment applications over 2018-2024
Publisher : Fractovia | Published Date : July 2018Request Sample
North America and Europe PEGylated drugs market has exhibited tremendous potential owing to the advancements in medical research and the prevalence of chronic diseases in developed countries that entail potentiated drugs for treatment. Unlike the parent drugs which have undesirable properties, PEGylated drugs are attributed with heightened solubility, reduced immunogenicity and extended circulatory time. The enhanced features of these drugs have encouraged R&D expenditures by developed economies towards effective PEGylation of biopharmaceuticals, boosting the North America and Europe PEGylated drugs market.
U.S. PEGylated Drugs Market, By Disease Indication, 2013 – 2024 (USD Billion)
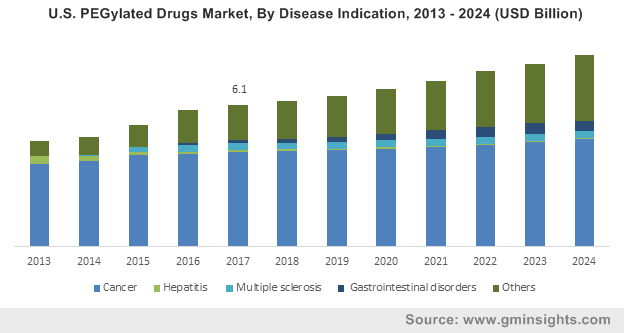
With the prominence of cancer, arthritis and other chronic disorders imposing the need for high efficacy medications, the North America and Europe PEGylated drugs market earned over USD 7.7 billion in 2017. Supporting the necessity for advanced therapeutic treatments are the statistics from the American Cancer society, which say that more than 15.5 million Americans are suffering from cancer with an anticipated 1.7 million new cases to be reported in 2018. Subsequently, developed nations such as the U.S. receive the largest share of investments in healthcare sector along with witnessing a spurt of biotech and biopharmaceutical start-ups. Phenomenal response has been garnered from government agencies and research organizations regarding acceptance of PEGylated drugs for improving the delivery of therapeutic treatments. Owing to the effective application in chemotherapy and availability of multiple FDA approved treatments, the North America and Europe PEGylated drugs industry is pegged as one of the most lucrative healthcare verticals, with cancer having contributed over 60% to the industry revenue in 2017.
To understand the implications of the cancer segment in defining the North America and Europe PEGylated drugs market, in 2015 about 1.3 million or 26% of all deaths in Europe resulted from cancer, making it the second biggest cause of fatalities in the EU. The upsurge in cigarette smoking and pollution along with unhealthy dietary consumption among EU population is expected to increase the frequency cancer and other chronic diseases. Consequently, the advantages of PEGylated drugs in improving treatments has inspired a number of companies in the region to develop bio-conjugated drugs, strengthening the North America and Europe PEGylated drugs industry.
Elaborating on the significance of North America and Europe PEGylated drugs market, a slew of PEGylated drug delivery systems such as Neulasta, Onivyde and PEG intron are employed in various cancer treatments, with additional drugs in the pipeline or pending approvals. For instance, the U.S. Food and Drug Administration (FDA) accepted a biologics license application from Shire PLC, a biotech leader in rare diseases, for Calaspargase Pegol (Cal-PEG) as a component of antineoplastic combination therapy for acute lymphoblastic leukemia (ALL) patients. The development is based on Shire’s research and experience with ONCASPAR (pegaspargase), a first-line treatment for ALL approved in the U.S. According to reports on the efficiency and safety of Cal-PEG, if approved it will permit availability of treatments that have a prolonged shelf life beyond the current PEGylated asparaginase treatment.
The advent of PEGylation to increase the power and life of drug induced therapies can be justified by explaining the impact of acute lymphoblastic leukemia (ALL). ALL is technically a cancer of the white blood cells, which leads to excess lymphoblasts in the blood, bone marrow and other locations in the body. The American Cancer Society estimates that the risk of developing the disease is highest among children below 5 years of age, which declines gradually by the mid-20s and then arises again after the age of 50. Further, ALL accounts for close to 75% of the overall leukemia cases in U.S. children and up to 78% for the children in Europe. As such, the obligation to produce potent medicines for combination therapeutic treatments has driven the North America and Europe PEGylated drugs industry.
There is a worldwide upsurge of biologic drugs in immunotherapies, advanced cellular and gene therapies, for which the ability of drug molecules to target specific cells or site is a prerequisite. In conjunction, numerous companies involved in the North America and Europe PEGylated drugs industry have been associated with the development and manufacturing of PEGylated drugs or provide PEGylation services. Citing an example, Bio-Synthesis, Inc. offers PEGylation and bioconjugation solutions for site-specific ligation of PEG to proteins/peptides, antibodies, lectins, toxics and drugs as a delivery system having several applications. The company claims to follow an exceptional approach to creating PEGylated population, in which specific PEGylation sites can be selected and controlled so that the efficiency is higher than other PEGylation techniques.
Speaking about notable biopharma companies in the North America and Europe PEGylated drugs market, Nektar Therapeutics is another research-based organization that develops and advances innovative medicine. It is believed that most of the FDA approved PEGylated drugs in the market were developed with Nektar’s PEGylation technology. As per company records, it has a vast number of licensing collaborations with biopharmaceutical giants across the world, and benefits from long-term revenue generating royalties from these partnerships. With foremost global biopharmaceuticals such as Amgen, Roche, Baxal and Bayer working in accordance with Nektar to expand and commercialize the portfolio of PEGylated drugs, the North America and Europe PEGylated drugs market is estimated to witness a CAGR of 5% over 2018-2024.